Dieter Finna (Click here for a German version)
For the printer, migration-optimized work is a discipline that serves to produce compliant products in packaging and label printing. Continuous control and documentation are essential throughout production. An overview of the most significant regulations and the essential tasks of the printing service provider resulting from them, summarized in seven key points.
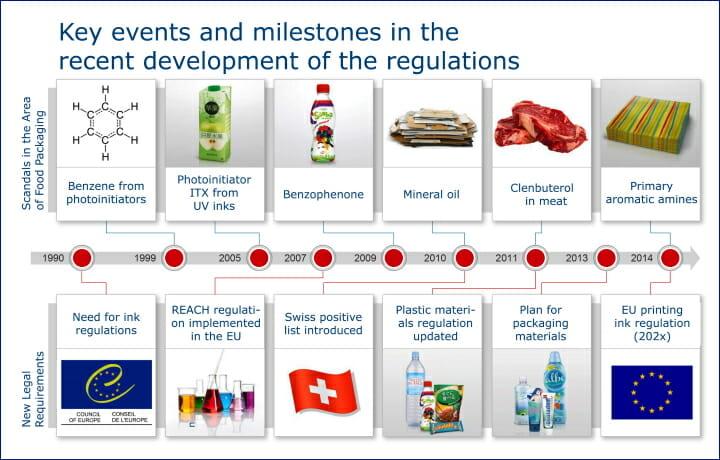
A look back at some key events in the history of food contamination shows why the topic of »migration-optimized working« is of the upmost importance for packaging today. Among the incidents uncovered in the past was the detection of the photoinitiator ITX in baby formula products in 2005 and, only a few years later, the discovery of benzophenone in food. Over the subsequent years further incidents followed, which were also reported on in the press.
Low migration or migration-optimized?
»Low migration« is often used as a term to emphasize that it is an ink system with a particularly low tendency to migrate. In this article, the term „migration-optimized“ is used instead, as this term describes this issue more precisely.
From an early stage, partly as a consequence of these events, the formulation of legal requirements and regulations began in order to better protect consumers from constituents of printing inks in food packaging. Three of today’s most important regulations were issued between 2004 and 2011 and form the basic framework of the regulations for packaging in consumer protection. In addition, but not as a direct consequence of the events mentioned above, the REACH regulation on the registration of chemicals came into force in 2007, which provided many new insights into input materials and their migration behaviors.
Conformity and traceability
As of 2004, Article 3 of the EU Framework Regulation (EC) 1935/2004 has stipulated that packaging that comes into contact with food must be constructed in such a way so as to ensure that it does not endanger human health. The regulation makes it compulsory for manufacturers of packaging materials to issue declarations of conformity for the products they produce and, based on this, to provide evidence of the conformity of the production steps. In particular, the obligation to provide evidence of traceability, i.e. of the substances or products used in the production steps themselves, must be observed.
 Good Manufacturing Practice
Good Manufacturing Practice
The GMP Regulation (EU) 2023/2006 obliges manufacturers of raw materials and packaging products to apply the »Good Manufacturing Practice« to materials and articles intended to come into contact with food.
The regulation requires manufacturers to have a quality assurance and quality control system ensuring permanent monitoring of the implementation of good manufacturing practice, as well as its documentation. The materials used in production must be selected so that they comply with the specification defined by the customer.
As part of a package, labels can also fall into the category of food contact materials and are then subject to the relevant regulations.

Migration limits
The Regulation No. 10/2011 on plastic materials, updated in 2011, lays out the basic rules for the production of plastic materials and articles. Since printing inks are directly connected to plastic films, their scope also extends indirectly to the printing inks used. The regulation sets the total migration limit (OML) at 10 mg /dm2 regardless of the pack size. In the case of cubic packaging, this corresponds to a migration of 60 mg/kg of food. The maximum value of non-evaluated substances is 0.01 mg/kg (10 ppb). Specific migration limits (SML) of approx. 1000 substances are listed in the appendix of the »Plastics Regulation«. Proof of conformity is required at every stage of production, which can be carried out by means of a migration test or via a model calculation.
Positive lists
In addition to the three European regulations listed, national regulations such as the Swiss Consumer Goods Ordinance 817.023.21 are of great importance for the packaging industry. Since it came into force in 2010, it has influenced other regulations in many respects, especially in the determination of migration limits. The positive list of substances used in printing inks contained in Appendix 10, which is regularly updated in line with the latest state of knowledge, has attracted considerable attention.
In addition to the legal framework, some brand owners have their own requirements for printing inks. One of the best known examples is the Nestlé Guidance Note on Packaging Inks with extended requirements through Nestlé-specific positive and negative lists.
Risks of a component transfer
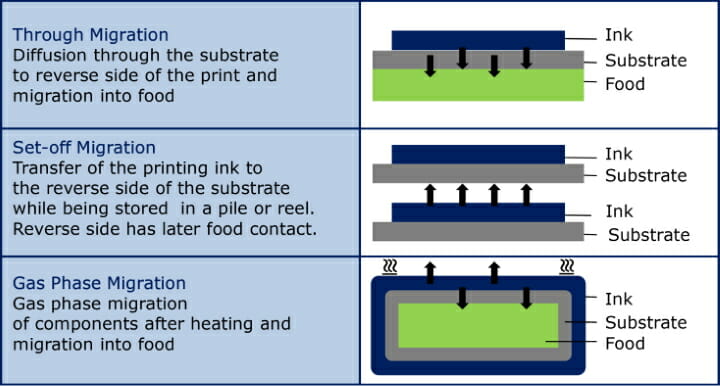
The way in which substances which are harmful to health can be transferred to foodstuffs is set out in the annex of the GMP Regulation. The risks of substance transfer to food are by, through migration, set-off or gas phase migration.
The role of the brand owner
Branded companies, printers as well as food manufacturers have different roles in the creation of packaging. These entities must cooperate with one another in order to prevent migration of substances that endanger human health into the packaged food. If a brand owner intends to launch a new product, they define the packaging and its specification. This is done according to the type of product and its consistency as dry, pasty or liquid food. The consistency and type of the filling material have a major influence on whether and how migration-capable components from the packaging can pass into it.
The criteria »storage period and storage conditions« are also taken into account in the specification. Processes after packaging, for example heating for pasteurization or sterilization, or heating in a microwave or oven, are also included in the requirement profile.
With the packaging structure, the brand owner determines the type of packaging, i.e. it determines whether the packaging is flexible or rigid, and among other things, whether a label is used. For this purpose, the branded manufacturer defines material properties such as the necessary material thickness and the required barrier properties.
In packaging design, i.e. the graphic design of the packaging, the brand owner determines the ratio of the surface to the filling material, but also the ink coverage and the total amount of ink to be applied. In doing so, they define key influencing factors for migration behavior. In the exchange of information along the value chain, it is necessary to communicate all of this information so that the packaging manufacturer and packer can record and take it into account.
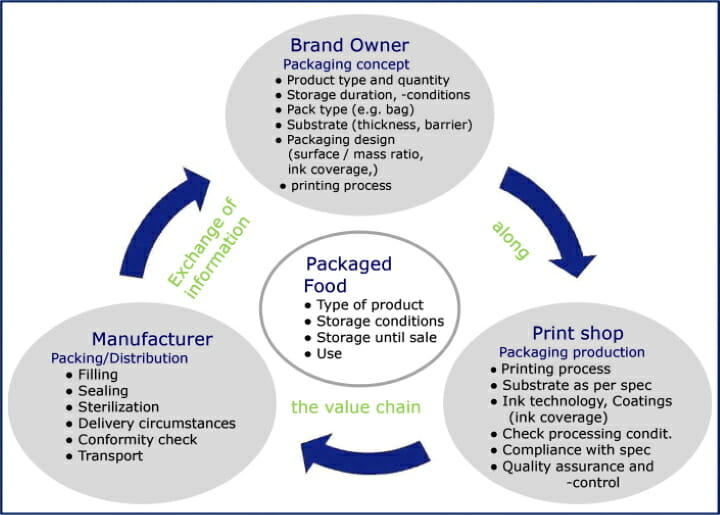
The role of the packaging and label printer
The print shop takes the requirement profile of the packaging from the specification of the brand owner and translates it into a process. In doing so, it determines the printing process, the substrate with its barrier layer properties, the appropriate ink technology and all suitable materials. In the prepress stage, the print shop can also exercise a limited influence on the amount of ink transferred by specifying the color separation for the production run. The migration risk from ink coverage and ink film thickness must comply with the specifications set by the brand owner.
The print shop receives a Statement of Composition (SoC) from its ink supplier, which contains information regarding the substances in the delivered inks which can potentially migrate and which are to be looked for in later analytical tests or which have to be evaluated. The data from the ink supplier together with the data from the glue and film manufacturers provide an overall picture for the migration potential of the materials used.
Control of the processing conditions
»The aim of the GMP-compliant manufacturing process by the printer is to limit and control any potential sources of contamination«, says Thomas Schweizer, Head of Product Management at Gallus Ferd. Rüesch AG, describing the requirements for print shops.
For the print provider, this means in concrete terms:
- For this purpose, the printing machine that is to be used exclusively for migration-optimized orders must be specified, as well as all permissible materials in a material list. This list includes all printing inks, coatings, glues, additives and cleaning agents used.
- The migration-optimised printing inks used must be stored separately to avoid confusion or contamination with conventional inks.
- In the maintenance log of the printing press, the printer ensures that quality-affecting machine parts such as the reflectors of the UV drying unit are cleaned regularly and that UV lamps are replaced at a determined point in time before they reach the end of their service life. In general, only approved solvents may be used for cleaning the press. All too often non-approved cleaning agents are found in the results of migration analyses.
- The printing speed used in production printing should be selected so as to ensure that the UV inks used are fully cured or that the specification for residual solvents is complied with.
- Compliance with these specifications is checked by the quality control department and
- documented in a quality assurance system ensuring that they can be made available on request.
- Quality assurance also includes the documentation of downstream processes of further processing such as offline lamination or the storage of intermediate and finished products.
»The aim of the GMP-compliant manufacturing process by the printer is to limit and control any potential sources of contamination«, Thomas Schweizer, Head of Product Management at Gallus Ferd. Rüesch AG
Declaration of conformity
In the value chain of brand owners, printers and manufacturers/distributors, the common principles do not allow for any unacceptable change in the quality, smell or taste of food caused by the packaging material. Confirmation of GMP-compliant production is ultimately provided by a declaration of conformity issued by the printing company for the packaging and label material. On request, the print shop must also be able to make evidence of compliance available detailing individual production steps for the purposes of traceability.
Migration tests
Migration tests or model calculations are a safeguard for the manufacturer to prove that the material supplied complies with the specification. For this purpose, print shops turn to specialized laboratories, which carry out such migration tests with specified simulants under defined test conditions. After a defined time of mass transfer, the simulant is analyzed in a gas chromatograph. It is important for the institute to be aware of which substances to look for. These are specified in the so-called »Statements of Composition« of the printing ink manufacturer. It should be noted that the information obtained by GC analysis is only valid for the sample being tested under the defined test conditions.
Migration-optimized production is teamwork
A low migration result can only be achieved through teamwork between brand owner, print shop and manufacturer. This illustrates the complexity of the value chain. All involved, from raw material suppliers to printing ink manufacturers, printers and food manufacturers, must follow the rules of good manufacturing practice. Ultimately, all of them, as distributors of their products, are responsible for ensuring that the specifications of the packaging produced are met and that consumers are not endangered by substances migrating from it. Compliance with these rules at every stage of production ensures that packaging material is produced in the value chain that reliably meets these requirements – »low migration« production or, »migration-optimized production« must therefore be seen as a discipline for all involved.
























